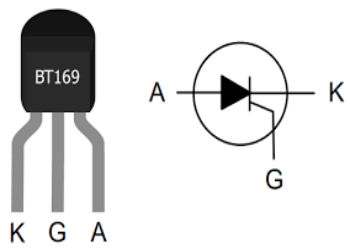
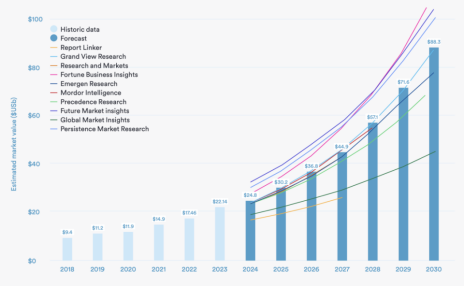
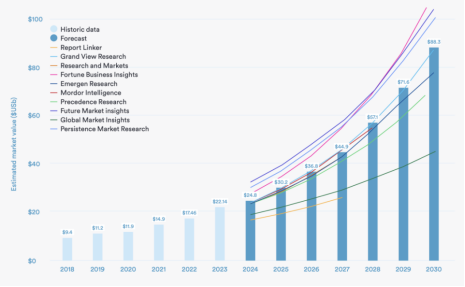
The relationship between the South-to-North Water Diversion East Route Project and BT169 thyristor
South-to-North Water Diversion Eastern Route Project is a large-scale water conservancy project in China that aims to solve the water shortage problem in the northern region through water transfer. BT169 thyristor, on the other hand, is a semiconductor device commonly used in power electronics to control and regulate current. In the first phase of the […]